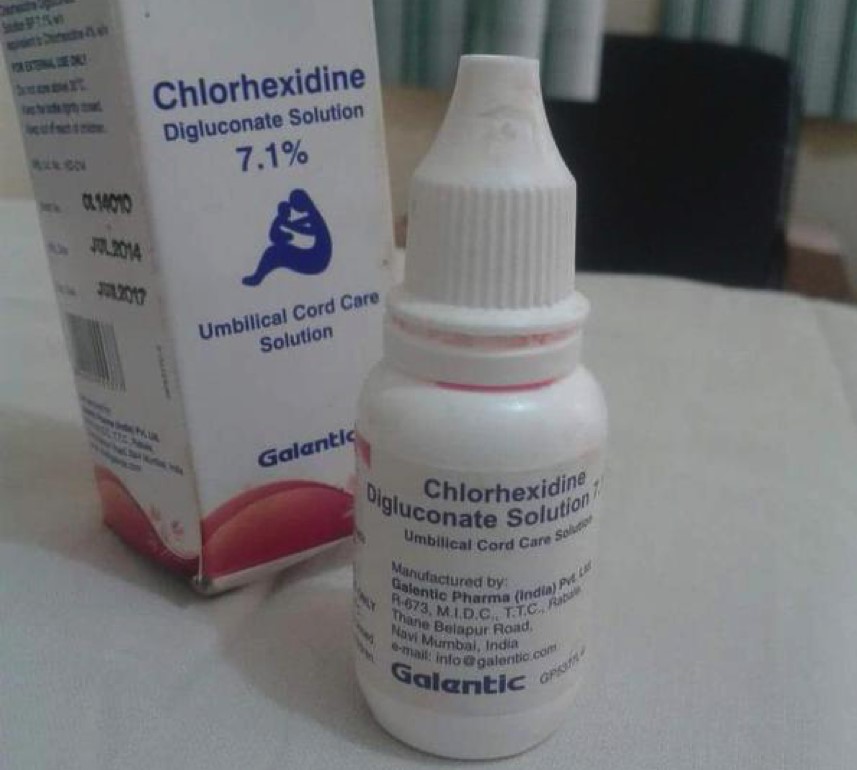
 On Sunday, September 6th 2015, a Nigerian health blog, Nigeria Health Watch, sent out a public announcement
concerning the misuse of Chlorhexidine digluconate 7.1% solution (CHX).
According the Nigerian Health Wathc, mothers have been using this
solution as an eye drop for their infants in Yobe and Adamawa states.
The CHX solution, originally used as umbilical cord infection treatment,
was packaged in an eye drop bottle and given to mothers as part of
their after delivery kit.
On Sunday, September 6th 2015, a Nigerian health blog, Nigeria Health Watch, sent out a public announcement
concerning the misuse of Chlorhexidine digluconate 7.1% solution (CHX).
According the Nigerian Health Wathc, mothers have been using this
solution as an eye drop for their infants in Yobe and Adamawa states.
The CHX solution, originally used as umbilical cord infection treatment,
was packaged in an eye drop bottle and given to mothers as part of
their after delivery kit.According to Nigerian Health Watch, there are already five known cases of irreversible blindness associated with the use of these ‘eye drops’, which has been in circulation since March 2015. Three children between the ages of three weeks to two years were reported to have gone blind in Yobe State as well as two children in Adamawa State.
The drug was approved by the World Health Organization (WHO) as part of the List of Essential Medicines for Children for umbilical cord care in 2013 .

Only 15 percent of Yobe Indigenes are literate
The Nigerian Bureau for Statistics estimated the literacy rate in Yobe to be about 15%, which means most of the women who got the ‘after delivery kit are not educated. Umbilical cord care is clearly written on the package of this solution, but because these women could not read and the bottle shape is similar to a typical eye drop container, they assumed that it should be used as such.
The solution does not have a NAFDAC number
The CHX solution does not have a Nigerian Agency for Food and Drug Administration and Control (NAFDAC) number. This means that NAFDAC did not test or approve these drugs before it was given out in hospitals in these two states.
This is what NAFDAC has to say:
 NAFDAC NIGERIA
NAFDAC NIGERIA
The drug Chlorhexiden was brought in by UNICEF as part of the kit given to mothers in an on-going UNICEF project in the two states.
NAFDAC NIGERIA
This sad incident is as a result of ignorance on the part of the affected mothers. Chlorhexiden is not a bad drug in itself
Government’s response after the announcementIn response to the news, the Federal Ministry of Health (FMoH) on Monday September 7th released a press statement. The ministry recommended that the State Ministries of Health and Primary Health Care Development Agencies and Boards should immediately withdraw and recall all inappropriately packaged Chlorhexidine solution for cord care from households, communities and health facilities.
1 comment:
Its pathetic situation oh....God go save us
Post a Comment